Corvus Pharmaceuticals Announces Positive Data from Cohort 4 Confirming Results for Placebo-Controlled Phase 1 Clinical Trial of Soquelitinib for Atopic Dermatitis
Cohort 4 data demonstrated positive safety and efficacy results, including additional clinical benefit observed following longer 8-week treatment
75% of soquelitinib patients achieved EASI 75, 25% achieved EASI 90 and 33% achieved IGA 0/1
Cohort 1-4 have demonstrated positive safety and efficacy results in patients who have received prior systemic therapy including patients who are treatment resistant
Company to host conference call and webcast today at 8:00 am ET / 5:00 am PT
SOUTH SAN FRANCISCO, Calif., Jan. 20, 2026 (GLOBE NEWSWIRE) -- Corvus Pharmaceuticals, Inc. (NASDAQ: CRVS), a clinical-stage biopharmaceutical company, announced positive results from cohort 4 of the randomized, blinded, placebo-controlled Phase 1 clinical trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis. The cohort 4 data demonstrated favorable safety and efficacy results consistent with results from cohorts 1-3, including a deepening of responses in cohort 4 over the 8-week treatment period compared to the 4-week treatment period. The results also showed clinical activity in patients who had received prior systemic therapies, including patients resistant to therapies like dupilumab and JAK inhibitors. The Company believes the data to-date also support the novel proposed mechanism of action with ITK inhibition, which is designed to act upstream and regulate multiple T cell functional pathways. The Company believes the immune rebalancing shown thus far by soquelitinib shows its potential in a wide range of inflammatory and immune diseases. Based on these positive results, the Company plans to initiate a Phase 2 trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis that have failed at least one prior topical or systemic therapy in the first quarter of 2026.
“The results from cohort 4 increase our confidence that soquelitinib could become a leading oral therapy for the treatment of atopic dermatitis,” said Richard A. Miller, M.D., co-founder, president and chief executive officer of Corvus. “The results also support our hypothesis from cohort 3 that the longer treatment could achieve deepening of clinical responses. Bottom line, we believe this is a successful Phase 1 program and the results have become stronger the longer we treat, without compromising safety. We believe this bodes well for advancing soquelitinib to the next phase where we will further test soquelitinib in a larger patient population. Taken altogether, we believe the data indicate that soquelitinib has the potential to be an important new medicine for first line or later line therapy of patients with atopic dermatitis that could be among the most active approved or investigational drugs for this indication.”
Dr. Miller added, “The data to-date also supports the novel proposed mechanism of action with ITK inhibition, which is designed to act upstream and regulate multiple T cell functional pathways. The immune rebalancing indicated thus far by soquelitinib shows its potential in a wide range of inflammatory and immune diseases.”
Soquelitinib Cohort 4 Data from the Atopic Dermatitis Phase 1 Clinical Trial
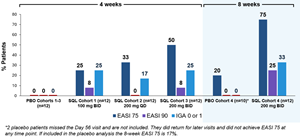
As of January 15, 2026, enrollment in cohort 4 was completed and all soquelitinib treated patients (n=12) had completed the 56-day treatment course. Of the 12 enrolled placebo patients, 10 were evaluable at day 56, as 2 patients were non-compliant and missed the day 56 visit; these 2 patients completed additional follow up at later time points. Patients in cohort 4 had similar baseline characteristics compared to patients in cohort 3, with patients in these cohorts having more advanced disease with a higher mean baseline EASI (Eczema Area and Severity Index) score compared to patients in cohorts 1 and 2. The mean baseline EASI in cohort 4 was 25.7 for the patients treated with soquelitinib and 21.9 for patients that received placebo. Across all four cohorts (n=72), 35% of patients had received prior systemic therapies (n=25), including 50% of patients (n=12) in cohort 4. Overall, all four cohorts showed meaningful responses in the soquelitinib treatment groups compared to placebo for clinically significant endpoints of EASI 75 and IGA (Investigator Global Assessment) 0 or 1.
Based on the encouraging results from cohorts 1-3, cohort 4 was prospectively designed to support and potentially extend the clinical results obtained in the initial cohorts. Cohort 4 patients were randomized 1:1 to receive soquelitinib (200 mg twice daily) or placebo and the treatment period was extended to 56 days from the 28 day treatment period used for cohorts 1-3.
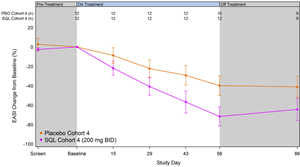
At day 56, the mean percent reduction in EASI for patients receiving soquelitinib in cohort 4 (n=12) was 72%, compared to 40% for patients receiving placebo (n=10). The kinetics of response demonstrated continuous improvement from day 28 to day 56 with widening separation of the response curve compared to the placebo. Two placebo patients experienced disease flares requiring therapy during the 56-day treatment period compared to none in the soquelitinib group.
Figure 1 below summarizes the efficacy results for cohorts 1 through 4 evaluating EASI 75, EASI 90 and IGA 0 or 1. Cohort 3 and 4 results appear similar, with cohort 4 exhibiting higher frequency of EASI 75 and 90. EASI 75, EASI 90 and IGA 0/1 for cohort 4 was achieved in 75%, 25% and 33% of patients receiving soquelitinib, respectively, compared to 20%, 0% and 0%, respectively, for the placebo group.
Figure 1: Percent Patients Achieving Endpoints EASI 75, EASI 90 and IGA 0 or 1 at Day 28 (cohorts 1-3) or at Day 56 (cohort 4) of Treatment.
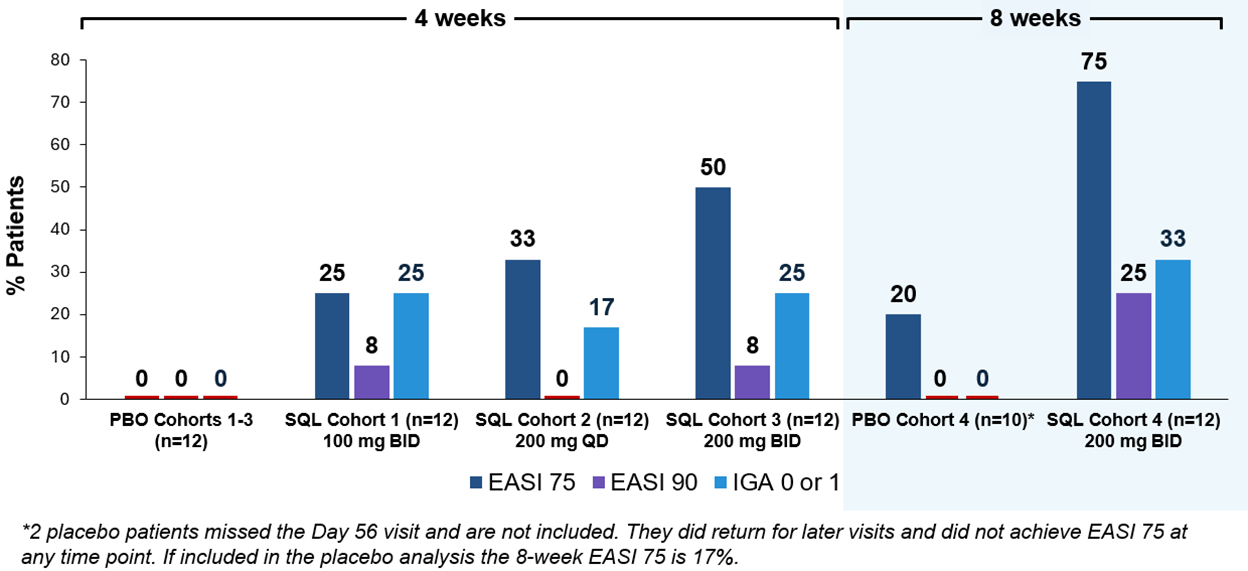
Figure 2 below shows the kinetics of response for cohort 4 patients receiving soquelitinib and placebo. Separation of the curves was seen at the first visit on day 15 and continuously increased out to day 56. At day 56, the difference in the curves is statistically significant, p=0.035. The disease control continued in the 30-day post treatment follow up period.
Figure 2: Percent Reduction in Mean EASI for Cohort 4. Mean percent change in EASI over time is shown. Treatment beginning is designated “Baseline” and days post-baseline are shown. Screening to baseline data is shown and demonstrates relative disease stability. The study blinding remained in effect for the entire 86-day period. Numbers at the top of the graphs indicate numbers of patients evaluated at the various time points. At this time, not all patients have completed the 30-day post treatment follow up.
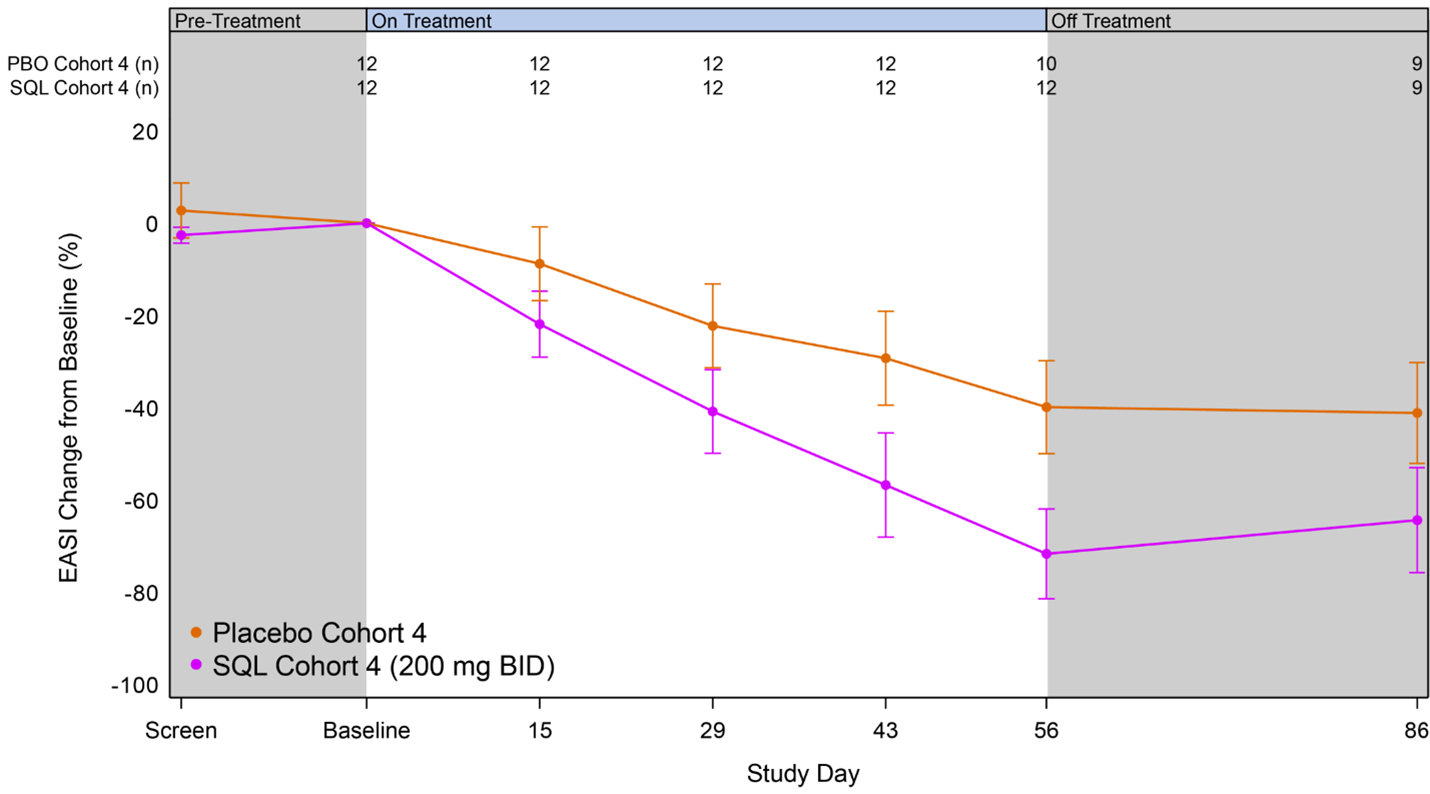
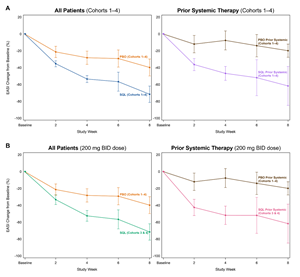
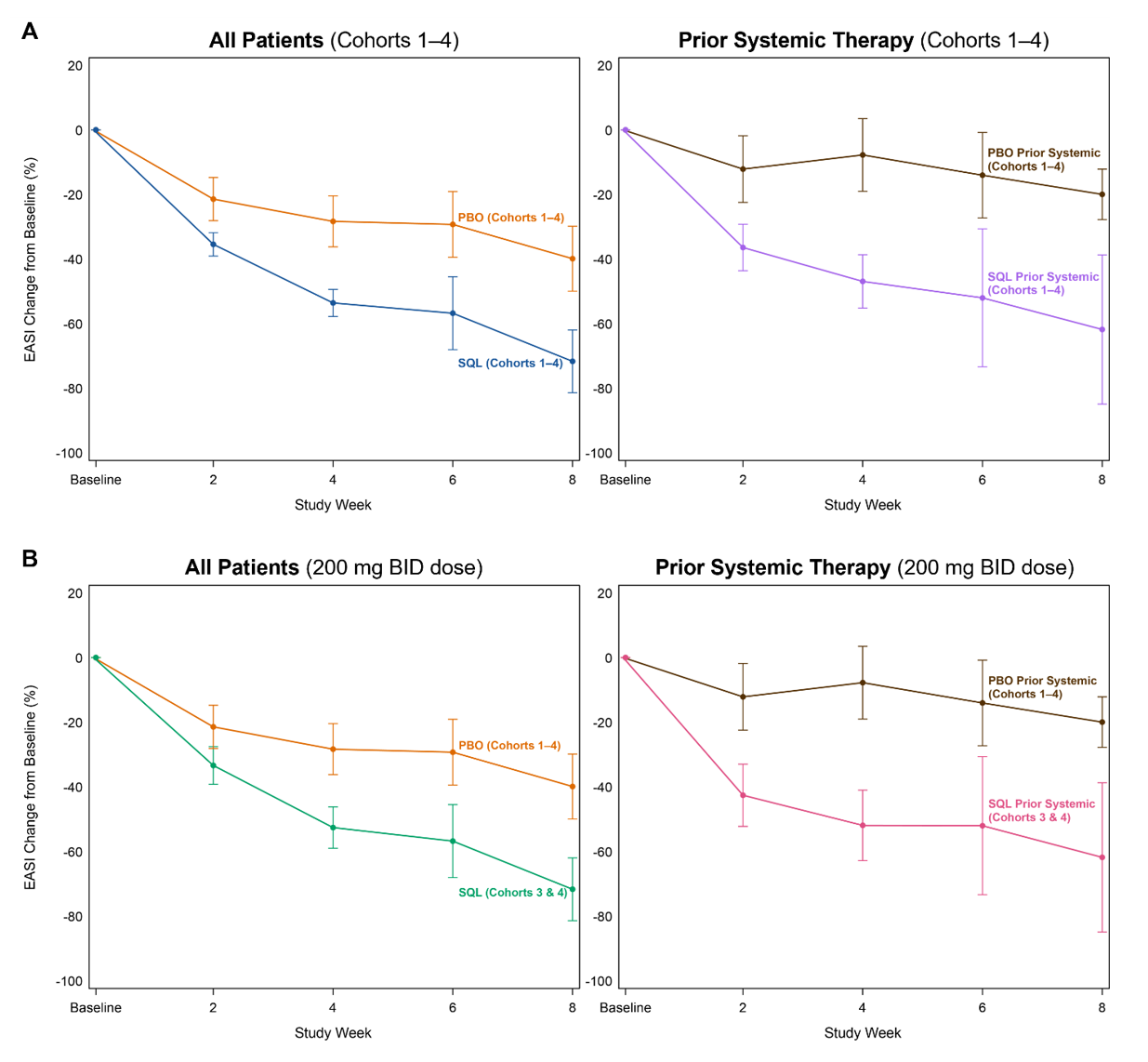
Figure 3A and 3B below show the response curves for soquelitinib and placebo patients across all patients in cohorts 1-4 and in patients in cohorts 3-4 only, also segmented into sub-groups by those who had or had not received prior systemic therapies. Across all four cohorts, 25 patients (35% of all patients) received prior systemic therapies with dupilumab being the most common prior systemic therapy (n=11). Other prior systemic therapies included JAK inhibitors, corticosteroids and investigational agents. The response curves are nearly identical for soquelitinib treated patients, regardless of their history of prior systemic therapy. Placebo patients who had prior systemic therapy had a lesser reduction in EASI compared to placebo patients who had not received prior systemic therapy. These Phase 1 results show separation of the curves for patients receiving soquelitinib compared to placebo and demonstrate that soquelitinib was similarly active in both systemic treatment naïve or experienced patients. Also, prior systemic therapy experienced patients appeared to have worse disease. The two placebo patients who achieved EASI 75 had not received prior systemic therapy. None of the seven placebo patients who had received prior systemic therapy achieved EASI 75 or EASI 50; whereas, three of the five soquelitinib treated patients who received prior systemic therapy achieved EASI 75.
Figure 3A, 3B: Percent Reduction in Mean EASI for Cohorts 1-4– Breakout of Patients with or without Prior Systemic Therapy. In Figure 3A, mean percent change in EASI over time is shown for all patients in Cohort 1-4 (left) and for those who received prior therapy (right). In Figure 3B, similar analysis is shown for patients in cohort 3 and 4; these patients received the 200 mg twice daily dose. In all charts, treatment beginning is designated “Baseline” and weeks post-baseline are shown. Placebo patients include cohorts 1-4. Soquelitinib cohorts 3 and 4 are combined (200 mg twice daily doses used in both of these cohorts).
Additional Analysis of Soquelitinib Cohort 3 Data
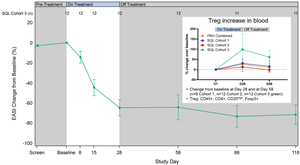
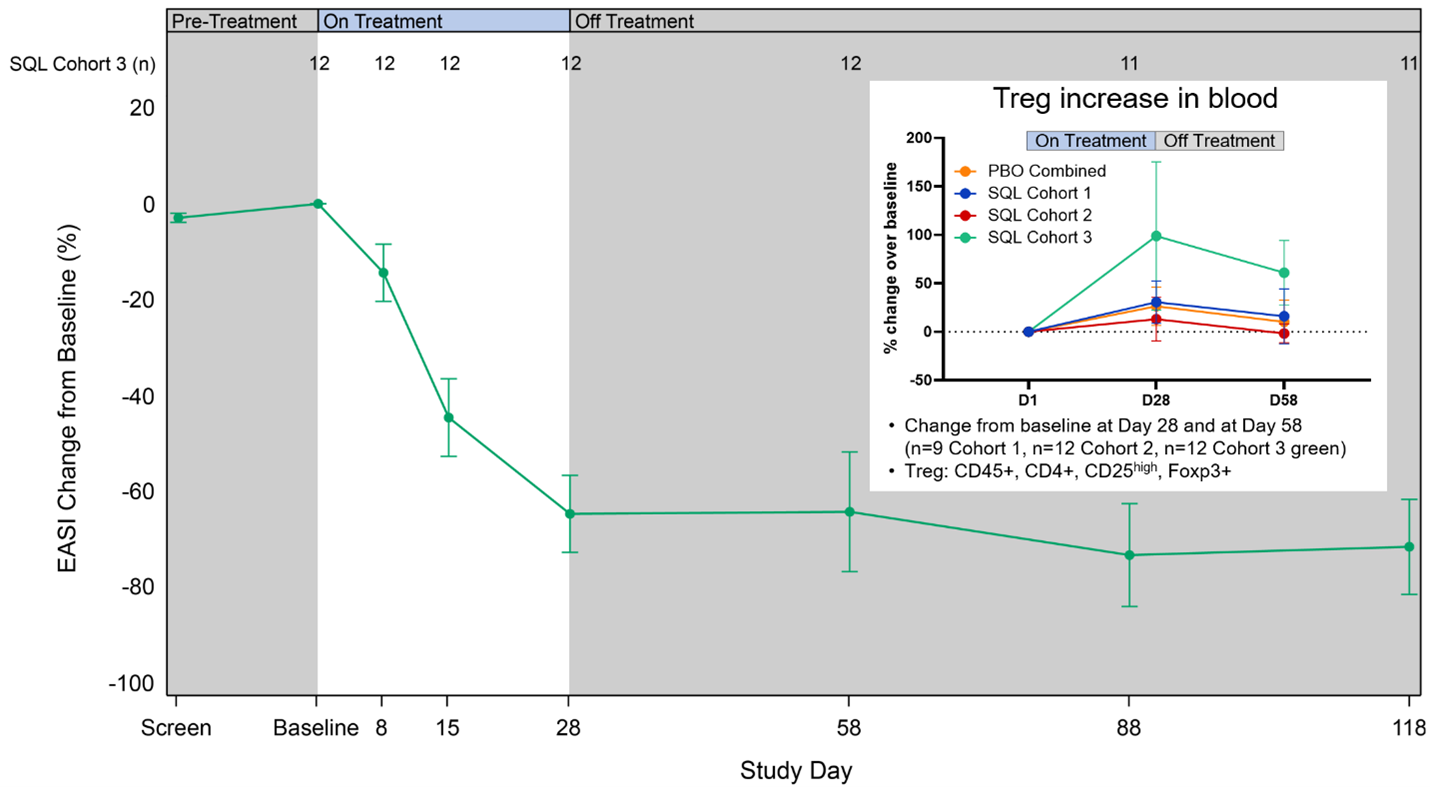
Figure 4 below shows longer follow up from cohort 3 patients, who also received the 200 mg twice-daily dose. The data show maintenance or improvement in EASI out to 3 months beyond the treatment period and an increase of circulating Treg cells.
Figure 4: Percent Reduction in Mean EASI for Cohort 3 and percent change in Treg cells for cohorts 1-3.
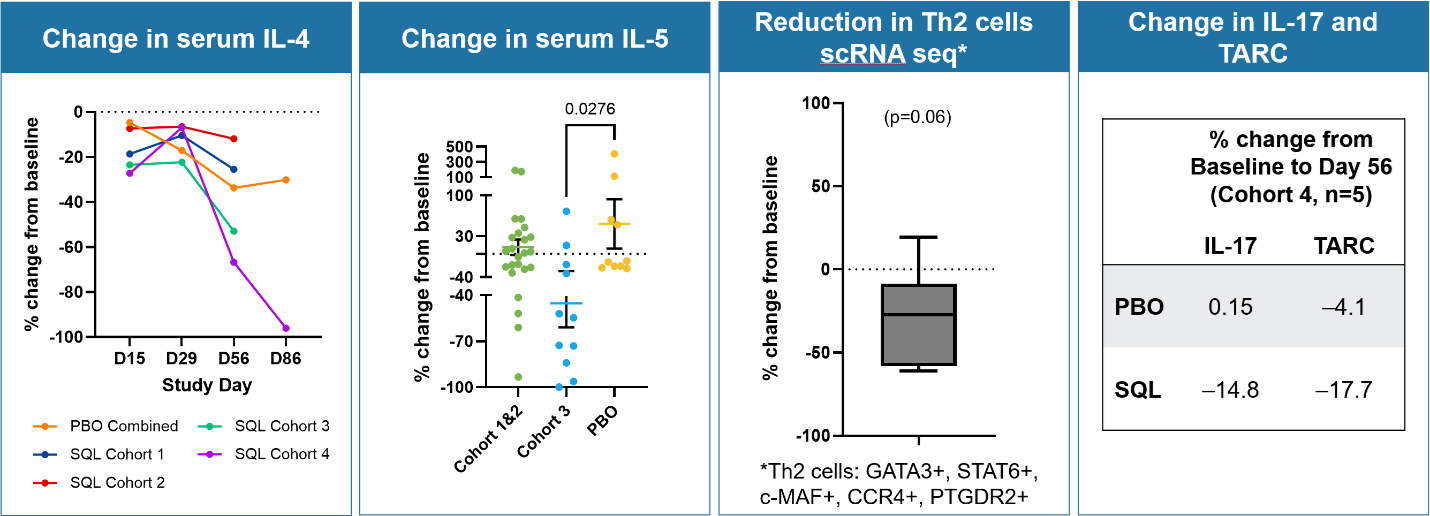
Biomarker Data (see Figure 5 below)
Reduction in serum IL-4 cytokine was observed in cohorts 3 and 4, both during the treatment period and in the post treatment period, including a dose dependent response with cohort 4 patients showing the largest reductions in IL-4 out to day 86. Biomarker data from cohorts 1 through 3 shows a reduction in serum IL-5 cytokine for cohorts 1 through 3 compared to placebo, including a dose dependent response with cohort 3 patients showing the largest reductions in IL-5 compared to placebo. The reduction in serum IL-5 occurred as early as day 8 of therapy. Serum IL-17 and TARC levels also were lower. Circulating Th2 cells were measured (n=6 cohorts 1 and 2) by scRNA seq technology and demonstrated a reduction in these cells with treatment. As noted above, circulating Tregs cells increased in patients in cohort 3 and were associated with a prolonged treatment effect. These results are consistent with soquelitinib’s observed ability to block Th2 and Th17 cells and their secreted cytokines, such as IL-4, IL-5, IL-17 and the effects on T reg cells. This biomarker data suggest that soquelitinib induced an immune system rebalancing involving Th1, Th2, Th17 and Treg cells.
Figure 5: Biomarker Data: Change in serum IL-4, IL-5, IL-17, TARC and reduction in Th2 cells. Percent change from baseline is shown for serum IL-4 (left panel). Percent change in serum IL-5 (middle-left panel) is shown for cohort 1 and 2 combined, cohort 3 and placebo. Each dot represents a patient. Percent change in Th2 cell is shown for six soquelitinib patients from cohort 1 and 2 that had scRNA seq performed on blood (middle-right panel). Change in serum IL-17 and TARC in cohort 4 for soquelitinib (n=5 measured to date) and placebo (N=14) patients (right panel).
Safety Data from Cohort 4
As of January 15, 2026, no new safety signals have been observed. Reported adverse events occurred in 41.7% of soquelitinib patients and 50% of placebo patients; all were Grade 1-2 and did not result in any dose modifications or interruptions. No severe or serious adverse events were reported. No significant lab abnormalities were seen.
Soquelitinib Atopic Dermatitis Phase 2 Clinical Trial
Corvus is on track to initiate a Phase 2 clinical trial of soquelitinib for the treatment of atopic dermatitis in Q1 2026. The trial is anticipated to enroll approximately 200 patients with moderate-to-severe atopic dermatitis that have failed at least one prior topical or systemic therapy. The trial is anticipated to enroll four cohorts of 50 patients each, with soquelitinib doses of: 200 mg once per day; 200 mg twice per day; and 400 mg once per day; along with a placebo group. The treatment period is anticipated to be 12 weeks with a 30-day follow-up period with no treatment.
Conference Call, Webcast and Presentation Slides
Corvus will host a conference call and webcast today, Tuesday, January 20, 2026 from 8:00 – 9:00 a.m. ET to provide an overview of the soquelitinib atopic dermatitis cohort 4 Phase 1 clinical data. The conference call can be accessed by dialing 1-800-717-1738 (toll-free domestic) or 1-646-307-1865 (international) or by clicking on this link for instant telephone access to the event. The live webcast, which will include presentation slides, may be accessed via the investor relations section of the Corvus website. A replay of the webcast will be available on Corvus’ website for 60 days.
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of immune diseases and cancer. The Company’s lead product candidate is soquelitinib, an investigational, oral, small molecule drug that selectively inhibits ITK. Soquelitinib is being evaluated in a registration Phase 3 clinical trial for relapsed/refractory PTCL and in a Phase 1 clinical trial for the treatment of atopic dermatitis. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com or follow the Company on LinkedIn.
About Soquelitinib
Soquelitinib (formerly CPI-818) is an investigational small molecule drug given orally designed to selectively inhibit ITK (interleukin-2-inducible T cell kinase), an enzyme that is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell immune function. Soquelitinib has been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of their secreted cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with cancers, including solid tumors, and in patients with autoimmune and allergic diseases. Recent third-party studies have demonstrated that ITK controls a switch between the differentiation of Th17 proinflammatory cells and T regulatory suppressor cells. Inhibition of ITK leads to a shift toward T regulatory cell differentiation, which has the potential to suppress autoimmune and inflammatory reactions. Based on interim results from a Phase 1/1b clinical trial in patients with refractory T cell lymphomas, which demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, the Company has initiated a registration Phase 3 clinical trial (NCT06561048) of soquelitinib in patients with relapsed/refractory PTCL. Soquelitinib is also now being investigated in a randomized placebo-controlled phase 1 clinical trial in patients with atopic dermatitis. A recent publication describing the chemistry, enzymology and biology of soquelitinib appeared in npj Drug Discovery in December 2024 and is available online at the Nature website and on the Publications and Presentations page of the Corvus website.
About Atopic Dermatitis
Atopic dermatitis, also called eczema, is a chronic disease that can cause inflammation, redness, scaly patches, blisters and irritation of the skin. It affects up to 20% of children and up to 10% of adults, and treatments include topical therapies, oral therapies and systemic injectable biologic therapies. It is frequently associated with other allergic disorders such as food allergies and asthma. Atopic dermatitis, like asthma and allergy, involves the participation of Th2 lymphocytes which secrete cytokines that result in inflammation. Soquelitinib has been shown in preclinical studies to inhibit cytokine production from Th2 lymphocytes.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to soquelitinib’s potential in a wide range of inflammatory and immune diseases, including atopic dermatitis; the ability of longer treatment with soquelitinib to achieve deepening of clinical responses; the potential safety and efficacy of the Company’s product candidates; and clinical strategy and the design of clinical trials, including the timeline for initiation, target or expected number of patients to be enrolled, dose levels, number of sites and other product development milestones. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the third quarter ended September 30, 2025, filed with the Securities and Exchange Commission on November 4, 2025, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of its product candidates; the accuracy of the Company’s estimates relating to its ability to initiate and/or complete preclinical studies and clinical trials and release data from such studies and clinical trials; the results of preclinical studies and interim data from clinical trials not being predictive of future results; the Company’s ability to enroll sufficient numbers of patients in its clinical trials; the unpredictability of the regulatory process; regulatory developments in the United States and foreign countries; the costs of clinical trials may exceed expectations; the Company’s ability to accurately estimate the cash on hand providing funding into the fourth quarter of 2026 and the Company’s ability to raise additional capital. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
INVESTOR CONTACT:
Leiv Lea
Chief Financial Officer
Corvus Pharmaceuticals, Inc.
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Sheryl Seapy
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com

Figure 1
Percent Patients Achieving Endpoints EASI 75, EASI 90 and IGA 0 or 1 at Day 28 (cohorts 1-3) or at Day 56 (cohort 4) of Treatment.
Figure 2
Percent Reduction in Mean EASI for Cohort 4.
Figure 3A, 3B
Percent Reduction in Mean EASI for Cohorts 1-4– Breakout of Patients with or without Prior Systemic Therapy.
Figure 4
Percent Reduction in Mean EASI for Cohort 3 and percent change in Treg cells for cohorts 1-3.
Figure 5
Biomarker Data: Change in serum IL-4, IL-5, IL-17, TARC and reduction in Th2 cells.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.